Our Mission
Cure Diseases
The Schisler Lab focuses on human diseases that are impacted by age-related effects. We study the mechanisms that control cellular metabolism and protein quality control, and how these components of our cellular machinery respond to both healthy and pathological aging. Our lab space is in the McAllister Heart Institute and we are proudly affiliated with the Department of Pharmacology and the Department of Pathology and Lab Medicine. We are located in the Medical Biomolecular Research Building.
Research
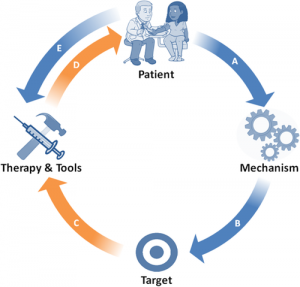
Our research program focuses on the mechanistic underpinnings of aging and the impact on human health and disease. In turn, we use these findings to develop pharmacological and genetic therapies. The expertise of The Schisler Lab is based in the integration of complex genomic-biologic data to elucidate risk relationships in both murine and human systems with a focus on human diseases that are mediated by changes in both metabolic and protein quality control mechanisms.
For example, we uncovered the first coding mutation in the gene STUB1 that causes a complex disease, most notably resulting in a profound loss in Purkinje neurons of the cerebellum. This results in cerebellar atrophy and profound ataxia. Two new genetic diseases are now recognized to be caused by coding mutations in STUB1. We are now using both genetic, computational, and pharmacological approaches to target both the protein encoded by this gene (the ubiquitin ligase CHIP) as well as substrates and de-ubiquitinases as therapeutics.
We are interested broadly in human diseases and have active projects that are rooted deeply in both the heart and brain, including neurodegeneration (cerebellar ataxias, Parkinson’s Disease, Alzheimer’s Disease), cardiac hypertrophy, heart failure, and accelerated aging. We integrate datasets derived from clinical genomics, transcriptomics, and metabolomics to generate hypotheses that we use to interrogate gene and protein function using a combination of atomic, molecular, cellular, and in vivo approaches. As we generate animal and cell-based models of health and disease, we continually go back to our clinically-derived datasets to refine our models.
Through the use and often combination of multiple ‘omic’ platforms, we performed several important studies on human diseases including a study that identified specific genetic variation between Caucasian and African Americans that modifies sugar metabolism genes contributing to diabetes and increased cardiovascular risk. We continue to expand our translational-based research program into other age-related diseases including coronary artery disease. The Schisler Lab actively collaborates with colleagues in the clinic and public heath to focus on extending translational research to at-risk populations where health disparities are prevalent and underrepresented in the literature. Our focus on aging has also led to collaborations with NASA to study how space flight impacts the age-related pathways we continue to characterize in our lab.